Dramatic Paragard Lawsuit Events Essential Updates and Insights
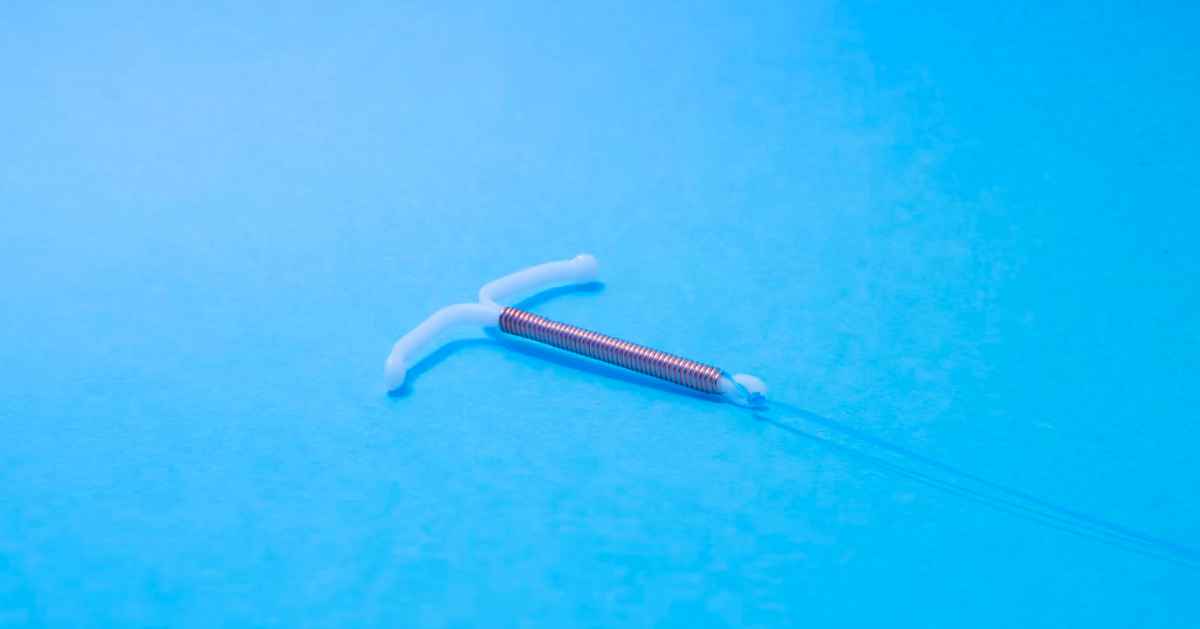
The concept of birth control is less than a century old. Over the past few decades, several devices (primarily intrauterine) have been introduced so that women may conceive as per their desire.
In 1988, a new intrauterine device (IUD) by the name of Paragard IUD appeared on the horizon. Initially, it had received Food and Drug Administration (FDA) approval for four years of usage. In other words, Paragard IUD was touted to be effective in preventing contraception for 4 years.
After the said period, it had to be surgically removed. However, data over the years revealed that the device was much more effective. Eventually, the FDA granted its approval for 10 years of implantation. This T-shaped copper-based IUD became widely popular in the early years of the 2000s.
However, women started filing cases of adverse events as the device was believed to have design defects. Many filed product liability lawsuits to receive financial compensation for their injuries. In this article, we will discuss the basics of the Paragard litigation along with its dramatic turn of events.
Why Was the Litigation Started?
The general procedure of implanting the Paragard IUD involves surgically placing it inside the uterus. This is said to prevent the sperm from fertilizing the egg.
The applicator is removed after device insertion, but its strings are trimmed short enough to avoid protrusion from the vagina. Some women had the Paragard IUD implanted within their body for as long as 8 years. At the time of removal, they were shocked to find that parts of the device had splintered or fractured.
According to TorHoerman Law, the most common injuries suffered by women include uterus perforation, excessive bleeding, ectopic pregnancy, pelvic inflammatory disease (PID), and injuries sustained due to device breakage.
Just like any other product liability case, plaintiffs of the Paragard IUD lawsuit have alleged that the manufacturers (primarily Teva Pharmaceuticals and Cooper Surgical) were aware of the risks involved. They chose to keep profits over people and continued to market the product as safe and effective.
The Plaintiff Fact Sheet (PFS) and Filing Fiasco
The Paragard IUD lawsuit is currently a class-action multi-district litigation (MDL) to ensure individual cases are settled fairly. This litigation was started back in December 2020, but the judge had not been paying much attention to it.
The progression has always been erratic, with the lawsuit becoming steady at some times and moving painfully slow at other times. Recently, a major development in the case has taken place. The judge had asked the plaintiffs to fill out a form known as the Plaintiff Fact Sheet (PFS).
Unfortunately, many failed to turn them in within the appointed time. The judge was not going to let the plaintiffs get away so easily. He concluded that those who hadn’t filled the PFS must provide a valid reason or drop their case.
After a thorough analysis, it was found that the deficient plaintiffs could be divided into the following six categories –
- Category 1 – Those who gave no reason for failing to meet the deadline.
- Category 2 – Those who agreed to have their cases dropped.
- Category 3 – Those who messed up their PFS and were unable to rectify the situation.
- Category 4 – Those where the attorneys could not find the plaintiff and the forms were also incorrectly filled.
- Category 5 – Those who needed more time to fill out their forms.
- Category 6 – Those who did turn up their forms but only after the court’s warning (which means they were late).
For the first four categories of plaintiffs, the court officially dropped their cases. As for the fifth category, the plaintiffs were granted 21 days from the date the order was issued. If they failed to turn up, such plaintiffs’ cases would also be dropped.
Only the final category’s forms were accepted. If we look closely, this is a promising development in the lawsuit. The PFS exposed cases that were not real lawsuits, and now the court can focus solely on cases that matter.
The Litigation’s Slow Progression
While the recent development sounds good, the Paragard lawsuit is still among the slowest litigation currently active across Federal courts. Given that the initial cases were filed in 2020, at least the first Bellwether trial should have taken place by now.
Even then, the former timeline for the trials (set for January 2024) was shifted further to October 2024. This means individual cases will need another year or two before their plaintiffs can expect fair payouts.
In the past few months, even the case volume has fallen. For the third month in a row (during November 2023), only 37 new cases were filed. However, the case selection process for the discovery pool has started taking place.
The Paragard lawsuit is a marvel of marvels with a constant dramatic turn of events. There are presently 2,283 cases awaiting settlements. Only the Bellwether trial outcomes will give attorneys a clear idea of the expected payouts for individual cases.
As of now, they are hoping that the litigation progresses steadily with no more roadblocks.
